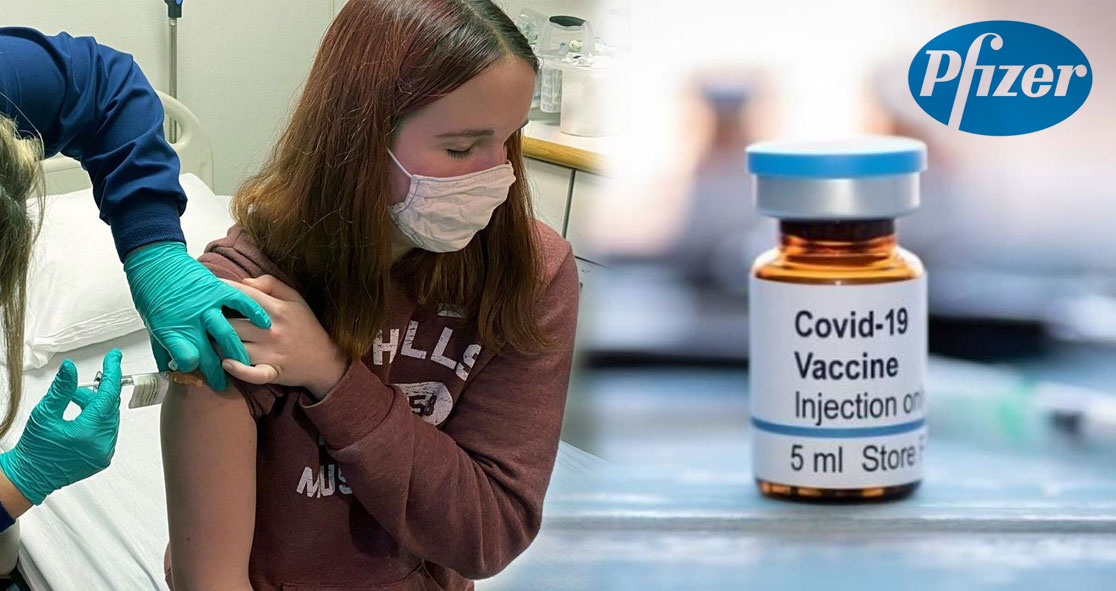Pfizer starts including teenagers in the Phase III clinical trials of its experimental COVID-19 vaccine, according to USA Today.
The company is expanding the participation age to high schoolers and middle schoolers to determine the safety and efficacy of the vaccine among these populations.
So far, Pfizer is the only company that is allowing minors to participate in its COVID-19 vaccine trials. Initially, the company lowered the age to 16, but this week, Pfizer vaccinated a 12-year-old in Cincinnati Children’s Hospital Medical Center.
According to USA Today, vaccine experts and pediatricians have voiced mixed reactions. Some experts said pharma companies should wait until the vaccines have been proven safe and effective in adults, while others said it is high time to proceed with vaccine trials among specific groups, including children and teenagers.
During an FDA advisory panel meeting, pediatric infectious disease expert Dr. Cody Meissner of Tufts Children’s Hospital said, “If I were part of the FDA, I would certainly want to be very convinced about the safety of a vaccine before I approved its use in children.”
He said the disease characteristics could be different in children, “which raises questions about testing both minors and adults at the same time.”
However, other experts argued that the ongoing pandemic calls for different protocols, which would get teens and children into clinical trials “as soon as possible.”
Dr. Barbara Pahud told USA Today, “We should not allow children to die. That’s our job as pediatricians to make noise and make sure people are noticing.” Dr. Pahud is an infectious disease researcher at Children’s Mercy Hospital, Kansas City, Missouri.
In the United States, over half a million children have contracted COVID-19 infection, according to USA Today. It is expected that more children might have been affected by the virus, but not tested.
Most children with COVID-19 recover without any major symptoms. However, some have died, with most of them getting the infection from their parents, grandparents, or teachers.
Dr. Robert Frenck, Director of Vaccine Research at Cincinnati Children’s Hospital, told USA Today, “With something that’s turned our world upside down, it gives you even a stronger reason of why you want to test now.”
Last week, Dr. Frenck gave Pfizer’s COVID-19 vaccine to minors and administered the vaccine on Thursday to the 12-year-old, the youngest volunteer so far.
He said he has seen a few side effects, such as fatigue, muscle pain at the injection site, and joint aches. USA Today reported that Pfizer has not released any details about the program for teens. However, the company said it would study the vaccine in one age group before conducting trials on younger ages.