NeuroSense Therapeutics, an Israel-based clinical-stage biopharmaceutical company, has developed an experimental drug called PrimeC to synergistically inhibit the progression of amyotrophic lateral sclerosis (ALS), according to Seeking Alpha.
The novel, extended-release (ER) oral drug is a fixed-dose combination of two generic drugs – ciprofloxacin and celecoxib – in a specific ratio.
The U.S. Food and Drug Administration (FDA) had approved ciprofloxacin (Cipro) to treat or prevent certain bacterial infections and celecoxib (Celebrex) to treat pain. Ciprofloxacin is an antibiotic, while celecoxib is a prescription non-steroidal anti-inflammatory drug (NSAID).
PrimeC is developed to inhibit the progression of ALS by regulating microRNA (miRNA) synthesis, influencing iron accumulation, and reducing neuro-inflammation – the hallmarks of ALS.
The FDA and European Medicine Agency (EMA) have granted PrimeC an orphan drug designation for the treatment of ALS.
In Phase II trials, the drug has been found safe and effective, allowing the company to plan a Phase III trial to further evaluate an upgraded formulation in two or three doses for ALS treatment. After the Phase III trial is completed, NeuroSense is expected to receive a patent allowance for PrimeC in the U.S.
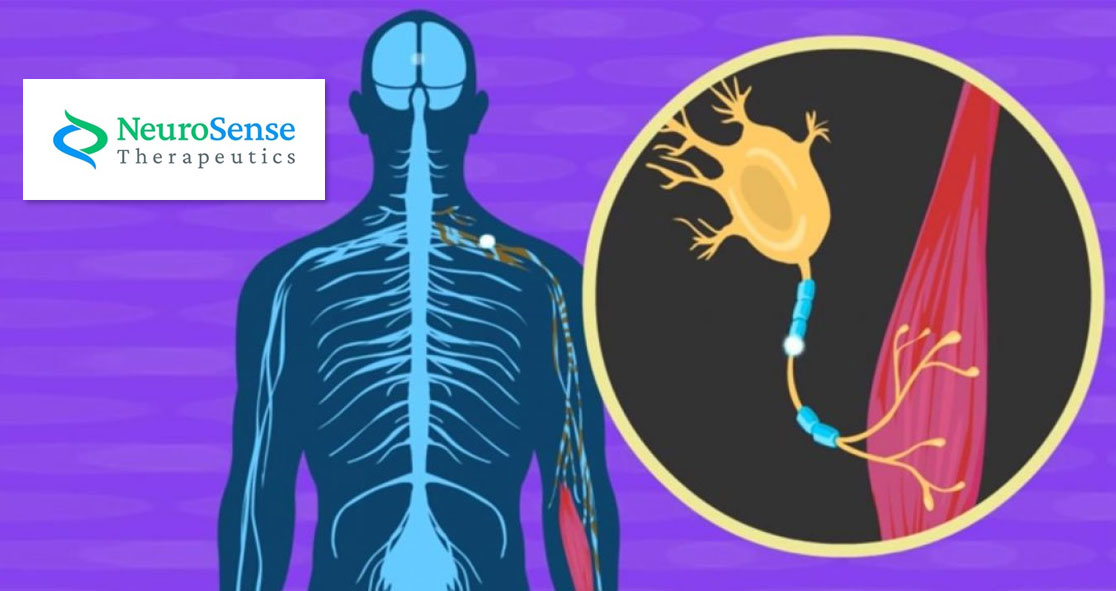
ALS is a progressive nervous system disease that affects nerve cells in the brain and spinal cord, causing loss of muscle control, according to Mayo Clinic. Often called Lou Gehrig’s disease, ALS often begins with muscle twitching and weakness in a limb, or slurred speech. Unfortunately, there is no cure for this fatal disease.
NeuroSense is a relatively new company with an experienced team at the helm to move forward with their new generic combo-therapy strategy resolving ALS as well as other neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease.
Founded in 2017 by Alon Ben-Noon, NeuroSense collaborated with world-renowned scientists and colleagues for the research and development of the experimental drug PrimeC for ALS.
In the United States, there is a substantial burden on patients with neurodegenerative diseases. Nationwide, ALS alone has an average annual cost of $180,000 per patient. In fact, it is estimated that ALS costs the U.S. healthcare system more than $1 billion annually.