The U.S. Food and Drug Administration (FDA) has announced the approval of Omnipod 5 Automated Insulin Delivery System for patients with type 1 diabetes, according to Medical Device Network.
The new system, developed by Insulet Corporation, is the first tubeless automated insulin delivery (AID) system.
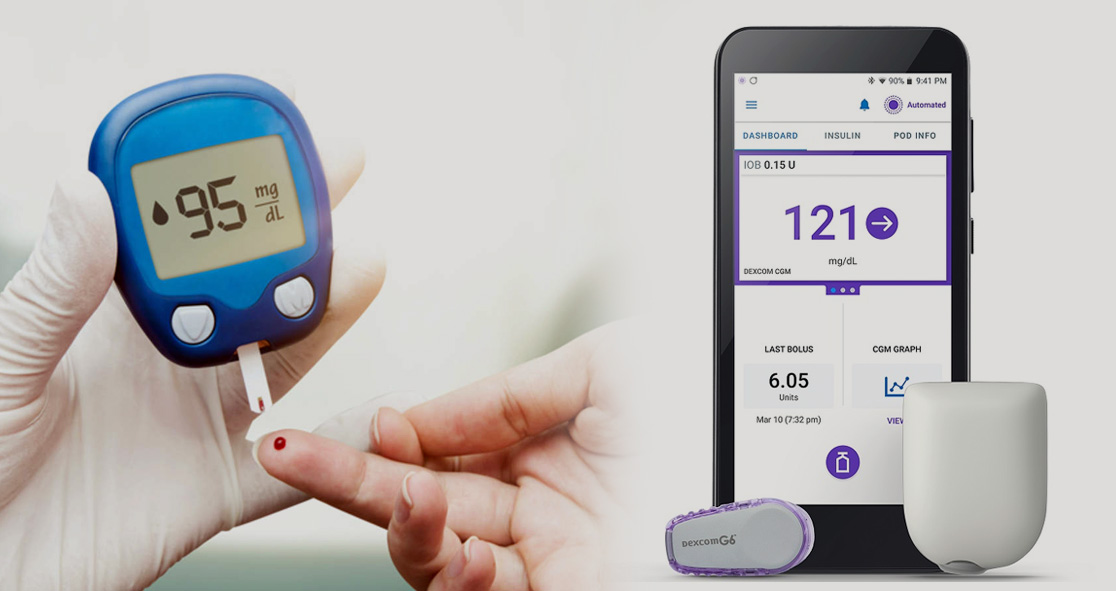
Omnipod 5 is available with a compatible smartphone and Dexcom G6 Continuous Glucose Monitor (CGM) System integration, which helps protect patients from variations.
It has a SmartAdjust technology that includes a tubeless insulin Pod, Dexcom G6 CGM, and the Omnipod 5 mobile app. In addition, it comes with an integrated SmartBolus Calculator.
Omnipod 5 is specifically designed to manage your blood glucose levels without fingersticks, tubes, or multiple daily injections.
Shacey Petrovic, CEO of Insulet, said, “Omnipod 5 is a life-changing technology that we believe will revolutionize the market and the lives of people with diabetes. We are incredibly proud of this simple-to-use, elegant system, designed to deliver unmatched freedom and to greatly simplify insulin management and improve glucose control for our users.”
The device users can download the app on their smartphones or use the Omnipod 5 Controller, which is available at no cost on the first prescription.
Insulet stated that SmartAdjust receives a Dexcom CGM value, tracking and predicting the users’ glucose level within the next 60 minutes. And then the Omnipod 5 system increases, decreases or pauses the insulin delivery using the patient’s desired and customized glucose target, protecting them from highs and lows.
The Massachusetts-based company will launch the system through the pharmacy channel. It will later release the system in a limited market, and shortly after that, the system is expected to be widely available in the market. In March 2021, Insulet reported positive results from the first trial of the Omnipod 5 Automated Insulin Delivery System.