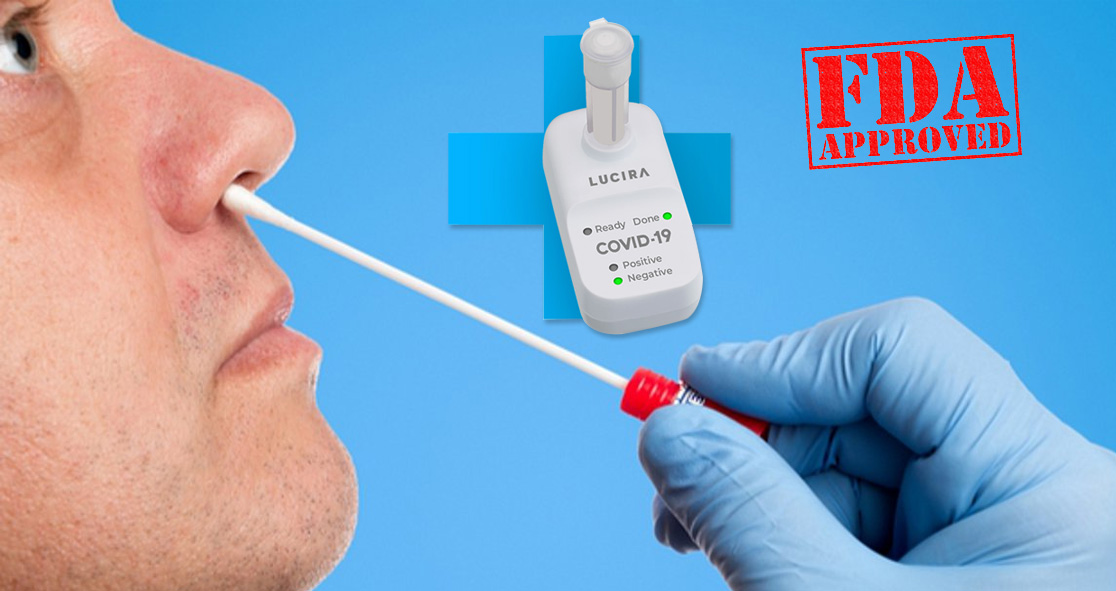
On Tuesday, the U.S. Food and Drug Administration (FDA) announced the approval of the first COVID-19 diagnostic at-home self-test that can offer rapid results.
The molecular single-use test, developed by Lucira Health, is an all-in-one test kit.
FDA Commissioner Dr. Stephen Hahn said, “While COVID-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home.”
“This new testing option is an important diagnostic advancement to address the pandemic and reduce the public burden of disease transmission,” he added.
The Lucira COVID-19 All-In-One Test Kit, which works by swirling a self-collected swabbed sample in a vial and placing it in a hand-held test unit, can offer results in as little as 30 minutes, according to the FDA.
The kit’s light-up display will show whether you are positive or negative for the novel coronavirus, aka SARS-CoV-2.
Until now, people have had to go to a doctor’s office, hospitals, medical centers, or some other sites for sample collection and analysis.
The home test will be available under a prescription to people above 14 who have symptoms similar to COVID-19 infection.
The FDA said patients under 14 could be given the test if it is administered by a healthcare provider.
Initially, the Lucira COVID-19 All-In-One Test Kit will be available on a limited basis in California and Florida. The test will be available nationwide by the spring.
Alex Azar, Secretary of the U.S. Department of Health and Human Services (HHS), said Tuesday that the new test adds “to our constantly expanding arsenal of COVID-19 testing options.”
“The Trump Administration has built the world’s largest testing system, and we will continue supporting both the development and manufacturing of cutting-edge options to make COVID-19 testing even easier and more accessible for the American people,” Azar added.
The country’s ability to combat the pandemic has been affected due to lack of testing and the recent spike in new cases has put additional strain on the nation’s precarious COVID-19 testing system.
Rob Stein, Correspondent and Senior Editor on NPR’s Science Desk, said, “Long lines are again forming in some places as the surge of infections drives a surge in demand for testing.”
“Testing companies, lab directors, and testing policy experts warn that waiting times for results could soon start to lengthen,” he added. “In fact, one of the largest commercial testing companies Tuesday reported turnaround times had already started creeping up.” The FDA’s approval of an at-home self-test kit could have a bigger impact on the testing routine throughout the nation.