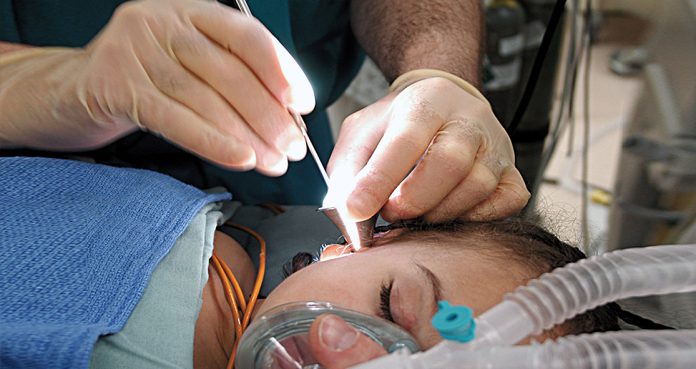
On Monday, the U.S. Food and Drug Administration (FDA) announced the approval of the first “tympanostomy” tube delivery system, which can be inserted into the ear under local anesthesia in a doctor’s office.
The system developed by Tusker Medical is called Tubes Under Local Anesthesia (TULA) system, which is recommended for inserting tympanostomy tubes for treating otitis media in adults and children above 6 months of age.
Otitis media is an inflammatory disease of the middle ear.
The TULA system includes the anesthetic Tymbion, tympanostomy tubes, and devices that are needed for delivering the ear tube into the eardrum under local anesthesia.
A small electrical current is needed to deliver the local anesthetic into the eardrum before inserting the tympanostomy tube.
The FDA approved the TULA system after considering the results of a clinical study conducted on 222 pediatric patients. The success rates of the procedure were 86 percent in children below five and 89 percent in children above five.
One of the most commonly reported adverse events was inadequate anesthesia while inserting the tube. Please note that the Tula system is not advised to patients below 6 months of age or patients allergic to local anesthesia. It is also contraindicated in patients with preexisting eardrum issues, including perforated eardrum.