Mezzion Pharma Co., a South Korean-based company, is confident about getting FDA approval of udenafil for the treatment of single ventricle heart disease (SVHD).
The company has already started the production at a pharmaceutical plant in Canada, according to Mezzion’s chairman Park Dong-hyun. He is optimistic that the drug will get FDA approval within the first half of 2021.
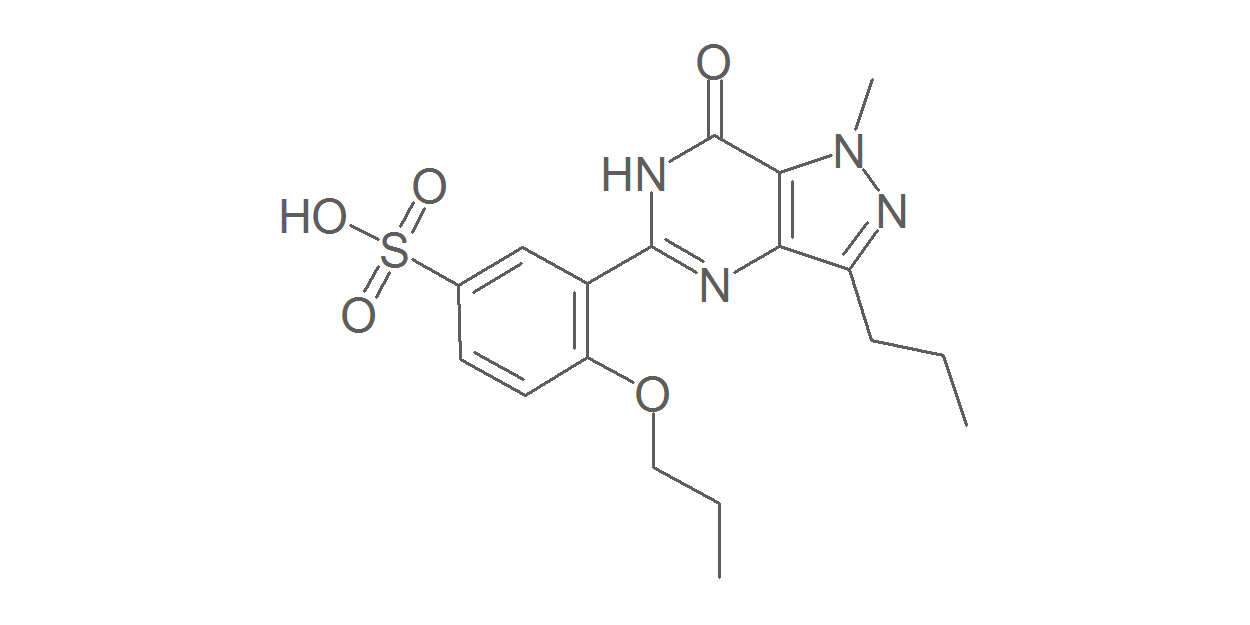
Like sildenafil (Viagra), vardenafil (Levitra), and tadalafil (Cialis), udenafil belongs to the class of drugs called phosphodiesterase type 5 (PDE5) inhibitors that are prescribed for the treatment of erectile dysfunction (ED).
Developed by Dong-A Pharmaceutical, udenafil is sold under the brand name Zydena for ED treatment; however, it has been studied as a potential drug for SVHD.
SVHD patients are treated surgically by separating the systemic and pulmonary circulations to improve oxygen levels and by redirecting venous blood directly to the lungs. However, this intervention is highly risky, which could even lead to cardiac death, requiring medical intervention.
On June 30, Mezzion submitted a New Drug Application (NDA) to the FDA for the approval of udenafil to improve the physiology of SVHD patients above 12 years after the surgical procedure.
Park said under the FDA’s review program, the entire process for regulatory approval will end in mid-March next year if the initial review is completed by mid-September. Even FDA experts are looking forward to the release of this drug as it is highly regarded as the first orphan drug for patients with single ventricle heart disease.
In the United States, SVHD is estimated to affect more than 25,000 patients aged 12 and above. Mezzion’s initial production of the drug is for 5,000 doses. Park said it has a dedicated sales team that will be set up in the United States where udenafil sales could reach up to $2 billion a year.
Park said that the timing of the drug launch for SVHD in Korea would depend on the FDA’s approval. The company is planning to start Phase I clinical trial to lower the age of population for udenafil treatment this year. Also, the company is planning to start a study to evaluate udenafil as a potential cure for the liver disease this month.