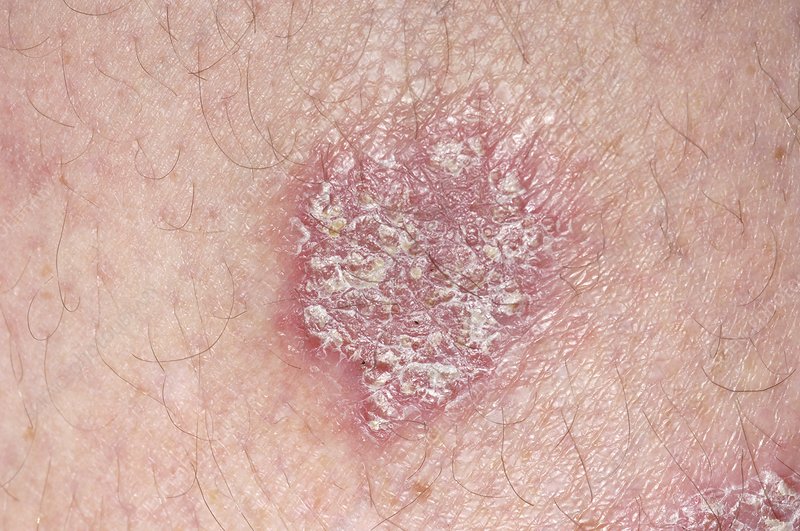
A clinical trial has found that netakimab, a humanized monoclonal antibody targeting interleukin-17A, is effective for the treatment of moderate to severe plaque psoriasis.
Researchers evaluated the efficacy of netakimab on the clinical features of psoriatic arthritis, including the activity of the disease, its skin manifestation, and quality of life.
The trial included 194 adult patients with psoriatic arthritis. Half of the participants received netakimab 120 mg and the other half received a placebo, subcutaneously for 24 weeks.
The researchers found similar baseline demographics and disease characteristics in both groups. Patients who were receiving a placebo, who did not meet 20% improvement of the American College of Rheumatology criteria (ACR20) by week 16, were switched to netakimab 120 mg.
The results have shown that 82 percent of patients who received netakimab 9 percent of patients who received the placebo achieved ACR20 at week 24. In addition, 70 percent of patients who took netakimab achieved ACR50.
The trial also found that patients with skin manifestations due to psoriasis, which negatively affects the quality of life, experienced a significant improvement after taking netakimab 120 mg for 24 weeks. More than half of the participants achieved complete skin clearance with the drug.
The researchers also found that netakimab sustained improvement in axial disease in patients who had inflammatory back pain.
The drug was well-tolerated throughout the study, and most side effects were mild to moderate. No patients experienced severe adverse effects with netakimab. Also, no anti-drug antibodies were detected.
In Russia, netakimab has been approved for the treatment of ankylosing spondylitis and psoriatic arthritis in spring 2020. The molecule of netakimab is based on the llama immunoglobulin, which is close to human immunoglobulins. It can help achieve a sustainable effect from drug therapy.