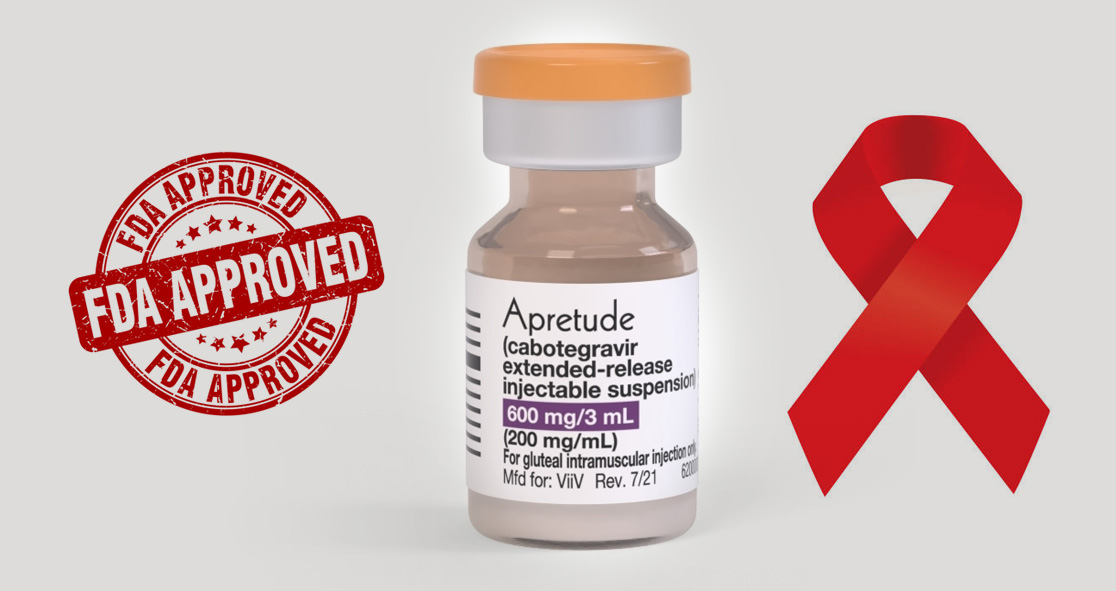
On Monday, the U.S. Food and Drug Administration (FDA) announced the approval of the first long-acting injectable medication for use as pre-exposure prevention (PrEP) against HIV, according to NBC News.
The new drug, called Apretude, is an injectable given every two months as an alternative to oral HIV prevention pills, such as Truvada and Descovy – the drugs that have been found effective at reducing the risk of HIV by 99% when taken daily.
The FDA analyzed two trials of Apretude and found that it was more likely to reduce HIV than the daily oral medications by 69% for cisgender men and transgender women who have sex with men and by 90% for cisgender women, according to the news outlet.
Dr. Debra Birnkrant, Director of the Division of Antiviral Products (DAVP) at the FDA, said, “Today’s approval adds an important tool in the effort to end the HIV epidemic by providing the first option to prevent HIV that does not involve taking a daily pill.”
“This injection, given every two months, will be critical to addressing the HIV epidemic in the U.S., including helping high-risk individuals and certain groups where adherence to daily medication has been a major challenge or not a realistic option,” she added.
The Centers for Disease Control and Prevention (CDC) says gains have been made in PrEP use over the past several years, but only 25% of the 1.2 million people for whom PrEP is recommended were prescribed the treatment last year.
As of 2019, approximately 285,000 people were using PrEP, the vast majority of them were gay and bisexual men, according to CDC.
Experts hope that Apretude, which is developed by ViiV Healthcare, will make adherence easier, increasing PrEP usage and driving down the national HIV rate.
ViiV CEO Deborah Waterhouse said, “People who are vulnerable to acquiring HIV, especially those in Black and Latinx communities who are disproportionately impacted in the US, may want options beyond daily oral pills.”
“Apretude was studied in one of the most diverse and comprehensive HIV prevention trial programs to date, which also included some of the largest numbers of transgender women and Black men who have sex with men ever enrolled in an HIV prevention trial,” she added.
Kenyon Farrow, Managing Director, PrEP4All, an advocacy group that fights to ensure that everyone can access HIV care, said his organization is “definitely happy to see the FDA approval of another option for people who want to use PrEP.”
However, he expressed concerns over the “implementation of this option will likely take years to make it real for most people.”
Farrow said in a mail, “Due to COVID, public health systems are already overburdened and much of the workforce needed to implement this large scale are leaving the field due to burnout.”
“Because it will need to be administered in clinical settings, it won’t be treated as a pharmacy benefit by payers,” he added. “But instead as a clinical benefit, which will take time to implement the proper coding for billing, as well as education and training for nurses who will likely bear the brunt of the work to implement.”